|
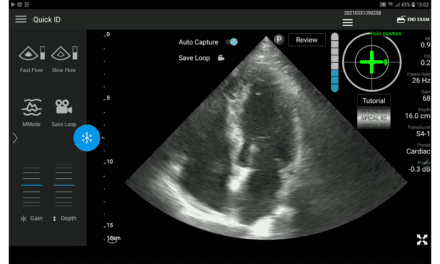
| This transesophageal ultrasound image of a mitral valve regurgitation in the heart was taken with GE Healthcare’s new Vivid i. For more information, see the “Have Power, Will Travel” section at the end of this article. |
Technology is a funny thing. As soon as it seems it can’t get any better, it does. The innovation that drives ultrasound is no exception.
“If you go back to the 1980s, people were saying, ‘We’ve pushed this technology close to as far as it’s going to go.’ Yet years later, we’re still pushing those limits,” says Franklin Tessler, MD, CM, professor and chief of body imaging in the department of radiology at the University of Alabama at Birmingham. “The trends we’re seeing are brought on by the application of faster computer processing to ultrasound equipment.”
As with every industry propelled by cutting-edge technology, ultrasound vendors are working to push the envelope by creating faster, smaller, smarter systems. October’s designation as Medical Ultrasound Awareness Month is the ideal time to inventory some of these advancements.
You Can Take It With You
From mobile phones to MP3 players, one sign of improving technology is a device’s shrinking size with expanding performance. The passion for ultrasound portability goes beyond simple convenience. The ability to take echocardiography into the intensive care unit or a physician’s office is redefining ultrasound’s role in modern healthcare.
“If you look at the high-end vendors, they seem to keep leapfrogging each other every few years,” Tessler says. “Some of the exciting stuff that’s going on is a result of that. It’s still a very technology-driven field.”
Indeed, vendors seem to be elbowing for room in a growing marketplace, all to the consumer’s benefit. Regardless of the desired use and size or even, to some extent, budget considerations, everyone can find a portable system to satisfy their needs.
Introduced in late 2002, the EnVisor system from Philips Medical Systems (Bothell, Wash) is a movable, cart-based ultrasound unit designed to perform whole-body imaging.
“It’s easy to move, and the level of imaging performance is near premium,” says Jim Brown, marketing communications manager of Philips Ultrasound (Andover, Mass). “It can deliver full diagnostic capability in many applications, including radiology, abdominal, obstetrics, vascular, and cardiac.”
|
Customers also can choose from a variety of carts to house the system-including one that collapses for easy transport-or can purchase a docking station, which allows the system to be mounted with peripherals.
Taking into account the nonstop pace of technology, Biosound Esaote (Indianapolis) designed the MyLab 30 CV to keep up with a facility’s expanding needs.
“We wanted to give customers a small system with high performance that is scalable,” says Jim Chapman, director of product marketing at Biosound Esaote. “Improvements can be incorporated as upgrades are available.”
The system weighs less than 20 lbs, runs on Windows XP, and is equipped with a CD burner. Future upgrades include contrast applications, tissue velocity imaging, and mapping as well as transesophageal echocardiography.
GE Healthcare (Waukesha, Wis) offers a portable system, the Logiq Book, which provides the full functionality of the company’s premium ultrasound product in a laptop-size machine that weighs around 11 lbs. Recent releases incorporate new transducers, including an intraoperative vascular small-parts transducer. The company also recently introduced a mobile, ultra-lightweight system with the performance of a full-size machine. (Read more about the Vivid i in “Have Power, Will Travel” at the bottom of this article.)
Have a Heart
Echocardiography is one specialty that’s reaping particularly huge rewards from handheld ultrasound.
“I use it at the point of care, whether I’m in the coronary care unit or seeing a patient in my office. Basically it’s a living autopsy-it’s very immediate,” says Robert Siegel, MD, director of the cardiac noninvasive laboratory at Cedars-Sinai (Los Angeles) and professor of medicine at the University of California, Los Angeles (UCLA). Cedars-Sinai currently has more than 32 handheld ultrasound units in use.
“The power of the technology allows us to see inside the chest to make important diagnoses,” he said. “This just wasn’t possible before. It’s really quite incredible.”
Due to the increased use of portable ultrasound in cardiac units, many manufacturers are creating systems specifically targeted for that function.
“The MyLab 30 CV includes a full array of cardiac probes, from low to very high frequency, which is particularly good for pediatric exams,” explains Biosound Esaote’s Chapman. The system also includes linear probes for vascular and small-parts imaging.
And the recent expansion of the Titan from SonoSite (Bothell, Wash) was tailor-made for echocardiography.
“This latest release enables physicians to perform full echocardiography and vascular studies in the clinic or at the point of care, allowing for earlier diagnosis and treatment,” says Kevin M. Goodwin, president and CEO of SonoSite.
In addition to its functionality, the Titan was designed with longevity in mind.
“We realize that when people carry things long enough, eventually they drop them,” says Dave Willis, VP of product strategy at SonoSite. “So the system has a magnesium shell and transducers designed to survive a 30-inch drop on a concrete floor.”
Keep the Hardware, But Improve the Software
 |
| Researchers from the University of Giessen in Germany used two Acuson Cypress echocardiography systems from Siemens Medical Solutions to investigate changes in cardiac performance and lung function at extreme altitudes during an expedition to the summit of the world’s highest mountain, Mount Everest, in May 2003. This study on the effects of altitude on pulmonary conditions was enabled by the high-quality imaging features and portability of the 19-lb Cypress system. |
In a modality as rich with technology as ultrasound is, the reality is that no matter how affordable new systems might be, it isn’t always feasible to replace hardware to obtain new functionality. Many manufacturers are compensating for that, creating software that can help bridge the gap.
“VisiQuest creates new applications that improve a system’s performance,” said Nicole Reineke product manager of VisiQuest, which was created by AccuSoft (Northborough, Mass). “Instead of replacing the hardware, customers can add software that brings the existing equipment to the next level.”
VisiQuest can be loaded on to existing hardware (including laptops) and is compatible with a variety of UNIX platforms, Windows (2000 and XP), and Mac OS X.
The program allows users to add one more dimension to 4-D images. In addition to height, width, depth, and time, another element-sound, for instance-could be factored into the exam.
VisiQuest also is capable of region-of-interest (ROI) processing, and it possesses a “tear-away” feature, making it possible for images to be sent and viewed even on computers that don’t have the software.
Siemens Medical Solutions (Malvern, Pa) also recently introduced a new software package. Clarify is a vascular enhancement technology that works to improve macro and micro vascularization.
“In the past, sonographers would spend a lot of time adjusting gain controls,” says Bill Carrano, VP of worldwide marketing for Siemens’ ultrasound division. “With Clarify, once the sonographer sets up an ROI, a simple push of the button will prompt the program to clear out acoustic noises, reverberations, and artifacts.”
Currently available as an enhancement to Siemens’ Sonoline Antares platform, soon Clarify can be added to the Acuson Sequoia systems as well.
GE Healthcare’s Logiq 9 comes equipped with the Speckle Reduction Imaging (SRI). “SRI is a new software technology that reduces the speckle artifact inherent to all ultrasound,” says Bob Thompson, global marketing manager for GE Healthcare Global Diagnostic Ultrasound. “It provides a much clearer image and improves the overall contrast.”
SRI is part of GE Healthcare’s TruScan software architecture, available on all Logiq systems. The software boasts several new features, including an advanced raw data archival system, which allows users to return to a saved image and make necessary changes without having to rescan the patient.
Technology With a Human Touch
Sometimes innovation happens at the expense of practicality.
“I think one thing all vendors completely ignored for many years was the fact that there’s a human being at the end of the transducer,” says the University of Alabama’s Tessler. “And I’m not talking about the patient; I’m talking about the sonographer or the physician doing the exam.”
Fortunately for those clinicians, as more became known about repetitive stress injuries and carpal tunnel syndrome, vendors worked diligently to incorporate ergonomic features into their ultrasound systems.
These adjustments aren’t merely superficial enhancements. In addition to saving time, they improve life in the echo lab.
“If [vendors] can do anything to improve the daily life of sonographers and make their work more comfortable and less likely to harm them,” Tessler says, “it’s at least as big a contribution as squeezing another bit of resolution out of an image.”
For starters, on many conventional ultrasound systems, menus are retrieved through various key combinations. As functionality increases, so do the necessary keystrokes.
“On the MyLab 30 CV [from Biosound Esaote], we’ve added soft key management, which ties software menus to individual hard keys,” Chapman says. Depending on what function is being performed, the same button will display a different, corresponding menu. This approach minimizes necessary keystrokes and also allows for efficient software upgrades.
“If the functionality is added to the system, we do it through the online menu so it isn’t necessary to change the hardware,” he adds. When the user presses the menu-display button, “the on-screen menu will instantly tell users what the new function is.”
The Logiq system from GE Healthcare also uses on-screen menus, and several of the company’s systems include touch-screen technology. The equipment line features the same software architecture on each machine, so clinicians who work on one Logiq system can work with them all.
|
Another user-focused improvement in ultrasound is incorporating wireless, hands-free technology. Two industry players are leading the way in this arena.
Philips’ iU22 ultrasound platform, launched in February, possesses voice-command functionality, enabling clinicians to adjust the system purely by speaking instructions into a wireless microphone.
“The voice-command feature reduces keystrokes and allows a lot more flexibility in scanning environments where clinicians don’t have a free hand,” says Philips’ Brown. “This is a new breed of machine.”
In October 2003, GE Healthcare introduced VoiceScan with its Logiq 9 system, giving sonographers control over more than 150 commands without touching the machine.
“We’ve done a lot of work with all of our systems around ergonomic improvements,” Thompson explains. “Voice command is key to portability and bedside exams.”
Also, the best image-capturing technology would be wasted without monitors capable of displaying a full range of detail. The advancements in flat-screen technology are being incorporated into many high-end ultrasound platforms.
Systems with large, adjustable LCD monitors are currently available from GE Healthcare (Logiq 9), Biosound Esaote (MyLab 30 CV), and Philips (iU22).
“This mobility allows the clinician to be in a neutral position while scanning,” Brown says. “In turn, that reduces stress and strain in the neck and the back.”
To provide additional user-focused improvements, GE Healthcare is rolling out several upgrades, including 4-D imaging for radiology use, to the Logiq 9 later this month. The system will include an abdominal transducer, gynecology probe, and a small parts transducer. The new Logiq 9 also provides sonographers with the ability to do multi-planar imaging, making normally out-of-range planes visible to aid in spatial location of legions.
New Uses for Existing Technology
As the cost of healthcare continues to climb, medical professionals are using ultrasound to improve procedures in an attempt to increase efficiency and avoid complications, limiting risk and expensive follow-up care.
According to Siegel, ultrasound-assisted line placement is quickly becoming an essential asset at Cedars-Sinai. “Using ultrasound for line placement is becoming the standard of care because of the safety of monitoring venous access. It makes it very easy,” he says. “It’s still evolving, but has been going on for more than a year here.”
The SonoSite iLook 25 was created to meet the needs of this growing trend. The system’s affordability and manageable size-the unit weighs a little more than 3 lbs-makes it ideal for use throughout a medical facility.
A Focus on Patients
In addition to turning their attention to the clinicians conducting exams, manufacturers are starting to think differently about the humans at the other end of the transducer-not only by improving the hardware, but also by improving how the system itself captures images.
One of the flaws inherent to conventional ultrasound is the system’s assumption that everyone’s physiology is fundamentally identical. In recent years, Siemens has focused on overcoming this issue with what the company calls patient-specific imaging. The first manifestation of this new approach is TEQ ultrasound technology, which offers individualized imaging with instant optimization for pulsed-wave and continuous-wave Doppler. TEQ is currently available on the Sequoia platform.
“The TEQ listens to the return ultrasound echoes, assesses multiple variables, and automatically adjusts the system parameters to optimize image quality particular to that individual,” says Siemens’ Carrano. “Not only does this improve image quality and consistency, but the automation also reduces repetitive stress injuries [by eliminating keystrokes].”
This constant process of feedback and modification creates an exam unique to each person. The system also works to reduce noise and improve resolution, and its advanced motion-detection capabilities adjust when the transducer is swept across broad regions-all without the clinician manually adjusting the gain control.
TEQ will be formally unveiled on the Sequoia platform at the 90th RSNA Scientific Assembly and Annual Meeting in Chicago, November 28-December 3.
“We agree that ergonomics are extremely important for sonographers using the system, and we’ve taken the concept to a new level,” Carrano says. “We have coined the term ‘ergometrics,’ ” which the company defines as the value a specific ergonomic feature brings to a product.
While cables and transducers can be improved with new designs, the team at Siemens thinks it’s also important to consider all factors affecting a sonographer, including each patient’s physical characteristics and the specific techniques involved with examining them.
As the percentage of overweight people in the United States increases, so does the technical difficulty for performing procedures on these patients in the echo labs. The increased amount of tissue means sonographers must apply more pressure to the transducer. Carrano says, “[It] improves the penetration, but it’s uncomfortable for the patient and not very good for the sonographer. With ergometrics, we’re looking at improving transducer acoustics.”
To help reduce the effort required by the technician, Siemens has incorporated the Hanafy Lens acoustic technology into many of the transducers on its Sequoia and Artares platforms. This technology produces uniformly thin image slices.
“The acoustic innovation is very important,” Carrano says. “We’re letting the higher level of efficiency of the acoustics work to the sonographer’s benefit. They push less, but get even more penetration than ever before-without the stress and strain on wrists, elbows, and shoulders.”
Without a doubt, vendors will continue to break technological barriers for years to come. Regardless of the innovations and improvements, the goal is always the same: to provide an ultrasound platform capable of delivering the best possible patient care.
“At the end of the day, it’s about the patient,” says GE Healthcare’s Thompson. “We need to make sure clinicians have the [right] tools.”
Dana Hinesly is a contributing writer for Medical Imaging.
Do the Means Justify the Ends?Is 4-D ultrasound filling photo albums-or is there more to it? When obstetric ultrasound became widely accepted in the 1970s, expectant couples eagerly awaited the ultimate first photo: a black-and-white profile of their child, still in the womb. While that anticipation hasn’t waned, gone are the days of parents squinting at fuzzy images in an attempt to differentiate one body part from another. The advent of 4-D technology provides a clear 3-D view of the developing fetus, in real time. In addition to its medical benefits, one result of this technology was the creation of on-demand imaging companies. These facilities bypass the medical aspect of ultrasound and use it strictly to produce the sepia-toned photographs and videos gaining popularity as keepsakes. But as this segment of ultrasound business has grown, so has the concern of those who question the safety of obtaining high-tech images solely for scrapbook purposes. Spearheading the effort to curtail this practice is the FDA (Rockville, Md), which acknowledges that the effects of multiple ultrasounds during pregnancy are unknown.1 It’s this uncertainty that is causing the greatest worry. Ill effects have not been proven, but the energy that ultrasound introduces into the womb is indisputable, and a number of studies indicate that it does affect the tissues and bones of a developing child. Exactly what that effect is, no one knows. For many, it is a risk not worth taking, and although ultrasound has been used safely for decades, the FDA emphasizes that it should be performed only by qualified professionals for medical benefit.1 In the 10 years since the FDA first learned of the keepsake-imaging trade, its position has not wavered. As recently as 2002, the organization warned that those conducting ultrasound for entertainment purposes are viewed as violating the law and that “persons who promote, sell, or lease ultrasound equipment for making ‘keepsake’ fetal videos should know that FDA views this as an unapproved use of a medical device.”1 The FDA isn’t alone in these convictions. In April 2004, The American Institute of Ultrasound in Medicine (AIUM of Laurel, Md) reiterated its opposition to the practice of medically unnecessary ultrasound.2 “The AIUM’s first concern is safety,” said AIUM President Lewis Nelson, III, MD, RDMS. “The amount of time required to produce a prenatal portrait that is visually appealing can take upwards of an hour, thus creating unnecessary exposure to the fetus.” As the FDA continues to battle entertainment-based ultrasound facilities, the agency has found an ally in legitimate commercial ultrasound organizations. Unlike keepsake facilities, these on-demand operations only perform exams with medical approval and purpose, with the touching memento simply a by-product. “We do not do ‘nonmedical’ ultrasound,” says Valerie Christensen, public education and media relations for Fetal Fotos USA (Salt Lake City). ” ‘Commercial ultrasound’ is not synonymous with ‘entertainment’ or ‘keepsake’ ultrasound. Our goal is to further reassure parents and bond them to their baby.” Christensen, who also is a sonographer and owner of four Fetal Fotos locations, explains that the company is still subject to-and has successfully passed-multiple FDA evaluations. “The medical review that the FDA requires is an integral part of our primary goal: to solidify the bonding experience between mother and baby, which will, in turn, create greater care for that baby,” says Leon Hansen, MD, FACOG, founder of Fetal Fotos USA. Based on studies indicating that 4-D technology helps parents connect more quickly with their child, Hansen feels that “on-demand ultrasound can have a dramatic, positive effect on prenatal care.” As the country’s largest provider of commercial ultrasound, Fetal Fotos employs only apprenticed, certified, and registered sonographers and adheres to all FDA guidelines. The company also obtains consent from each woman’s medical provider to ensure that the scan is not being used to replace prenatal care. “Ultrasound is a proven, noninvasive technology,” Hansen says, “that can positively invest the woman and her family in the pregnancy.” -DH References
|
Have Power, Will Travel
The introduction of handheld and portable technology to ultrasound has ushered the industry into a new era. The collective focus has shifted from achieving the best quality, regardless of the machine’s size, to having the same performance in a unit taking up a fraction of the space. The bad news is that in many cases, scaling ultrasound systems down to a manageable size means sacrificing something-whether it be speed or image quality. The good news? Technology brings with it a promise that the next best thing is never too far away. And it seems the next best thing is here, courtesy of GE Healthcare. Launched in late August at the European Society of Cardiology Congress (Munich), the Vivid i is the world’s first high-performance, portable cardiovascular ultrasound system. “In designing the Vivid i, we set out to tackle one of the biggest challenges in caring for cardiovascular patients: access to complete, real-time diagnostic information,” says Omar Ishrak, president and CEO of GE Healthcare’s ultrasound unit. “Now, we can help expand the reach of echocardiography by giving clinicians the freedom to use advanced diagnostic ultrasound technology virtually anywhere.” GE Healthcare expects the system to establish a completely new standard for clinical performance and mobility in the field of echocardiography. “With the Vivid i, we’re introducing a new generation of cardiovascular ultrasound systems,” says Al Lojewski, global marketing manager of cardiovascular ultrasound. “This product provides the high performance of a full-size system, in a mobile, ultra-lightweight design.” Even with portability in such demand, GE Healthcare doesn’t consider size to be the best feature of the Vivid i. “We don’t think of it as a handheld product,” Lojewski explains. “It’s a full-featured, high-performance system that just happens to be portable.” Though it weighs in at less than 11 lbs, the list of features is definitely not lightweight. The Vivid i includes a 15-inch LCD screen, full diagnostic capabilities, an archive system, DICOM capability, and wireless networking options, along with a CD burner and a USB port. Fully charged, the battery will power at least an hour of scanning. Still, size does matter. “It is no longer necessary to bring the patient down to the echo lab for a high-performance echocardiography exam,” Lojewski explains. “This system can take that level of exam to the patient-wherever they are.” Early feedback from physicians indicates that the Vivid i is ideal for transesophageal echocardiograms-allowing close monitoring of a patient during surgery without having to squeeze a conventional ultrasound system into the operating room. The system’s technological adaptability also means that if a problem is detected, images can be sent instantly to the cardiologist for a remote consultation using either a wireless or LAN network connection. “Whatever the customer wants-whether it’s to be wireless or wired, to be hooked to a PACS system or burn a CD, to use Bluetooth connectivity-the possibilities are limitless,” Lojewski says. “And to get that, you do not compromise screen size, portability, or performance.” -DH |