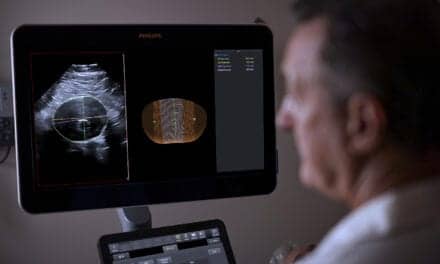
SoftVue, a whole breast ultrasound tomography system from Delphinus Medical Technologies, Inc, has received 510(k) clearance from the US Food and Drug Administration (FDA) for diagnostic breast imaging.
According to the manufacturer, while most ultrasound systems only capture echoes returning toward the linear transducer, the SoftVue system transmits and receives ultrasound signals traveling through the breast and from all directions surrounding it. Requiring the breast to be suspended in warm water and taking 1 to 2 minutes per breast, the system provides a holistic image not reliant on user experience or technique for clarity.
“We believe that the opportunity now is better than ever for SoftVue’s ultrasound system to advance the state-of-the-art in breast cancer diagnostics,” said Paul McCreadie, chairman of the board of Delphinus. “The shortcomings of existing modalities are widely known, and SoftVue’s ability to provide early and accurate diagnostic information without the risk of radiation exposure will serve to improve women’s health.”
For more information, visit Delphinus Medical Technologies.